18 November 2020
Do you want to know how the use of biomass can contribute to a more sustainable society?
PhD student Lukas Wolzak and Keimpe van den Berg (AkzoNobel) explain the purpose of their research on the basis of their recent paper ‘Titanium-catalyzed esterification reactions: beyond Lewis acidity’.
Lukas is a PhD student at the University of Amsterdam and performs his research about Polyester synthesis using novel and efficient esterification catalysts within ARC CBBC in collaboration with his industrial supervisor Dr. Keimpe van den Berg (AkzoNobel).

Within ARC CBBC, research is focussed on new chemistry to enable the production of sustainable energy and materials. In which way does your catalysis research contribute to a sustainable use of our resources?
Wolzak: The purpose of my research is to make the production process of polyesters, which are used as binders in paint, more sustainable. One way of achieving this goal is the use of a catalyst, which is an auxiliary agent enabling the reaction to be more efficient.
How is this process currently conducted at AkzoNobel?
Van den Berg: Currently we (and also other paint and resin manufacturers) already use a certain catalyst for the production of polyesters, but with a better catalyst the temperature, knowing that polyesters are produced at temperatures above 200 °C, of this reaction can be reduced.
What is the advantage of a lower reaction temperature?
Van den Berg: A lower reaction temperature has huge advantages. A lot of energy can be saved when polyesters are produced at a lower temperature. This results in a lower CO2 emission from the production of binders. In addition, there is a desire to make polyesters directly out of biomass. From biomass, different building blocks for polyester can be extracted than from fossil sources.
Wolzak: The use of biomass for making the building blocks of paint is very exciting. It gives you the opportunity to create completely new polyesters with novel characteristics, causing the paint to have other characteristics as well. An example could be self-healing paint. However, some building blocks for polyesters derived from biomass are not suitable for the high production process temperature. They decompose or discolour. A better catalyst would resolve this problem.
What are the challenges in the development of a new catalyst?
Wolzak: The development of a new catalyst, which is the purpose of my PhD research, is not an easy process. Because a new catalyst would imply so many advantages, many different materials (most of them of a certain type: Lewis acid) have already been tested for this reaction. Still there is limited knowledge to why this certain type of material is working well.
What does your paper tell us?
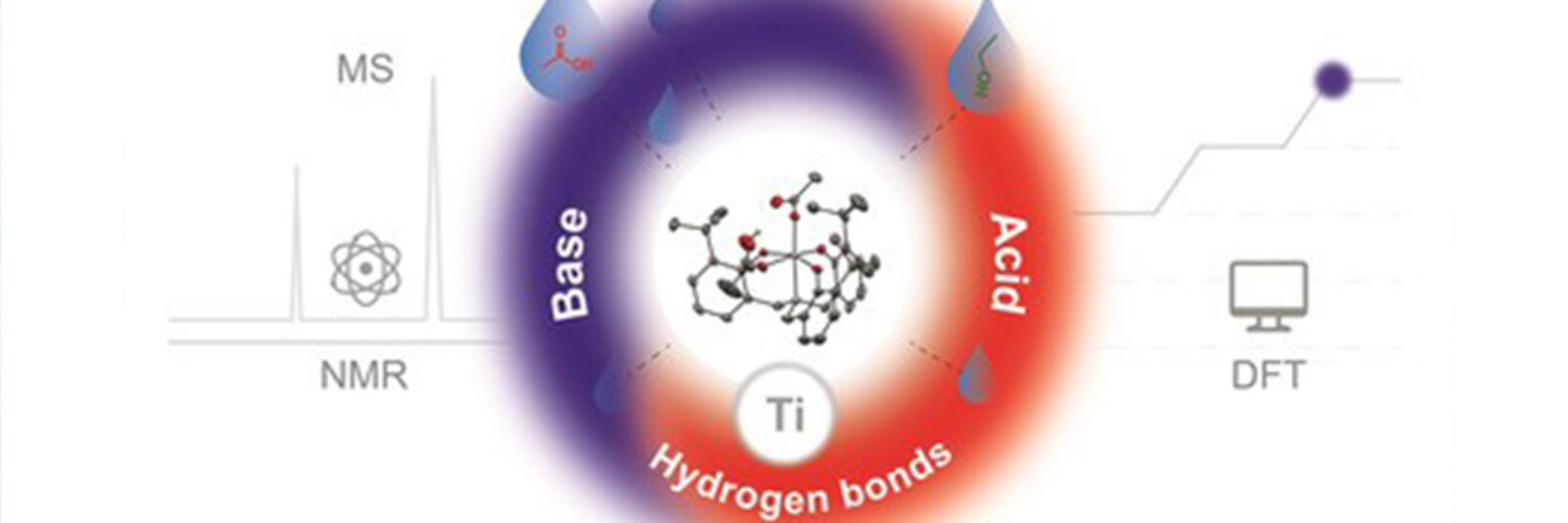
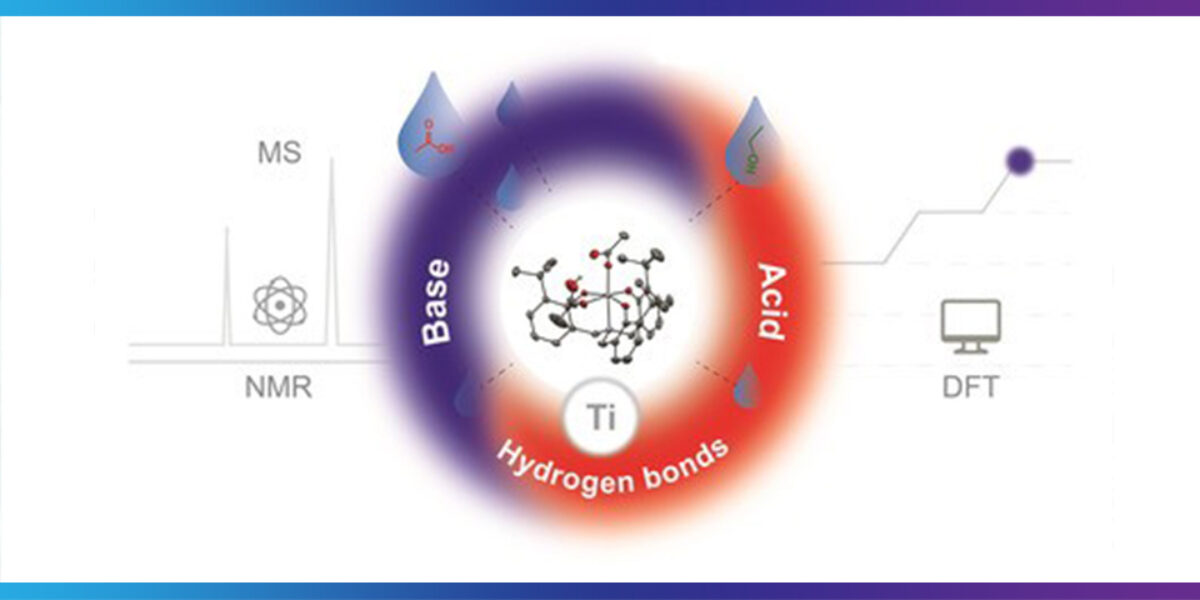
Wolzak: Our paper shows, using a model system, that there are three different characteristics of the catalyst which are important. Besides being a Lewis acid, it works as an internal base and it also can form hydrogen bonds with the substrates.
Van den Berg: In summary, we have studied the function of a catalyst when producing esters, which is the type of bonds that forms polyesters.
Wolzak: We have not found a better catalyst yet, but with the gained knowledge we still hope to be able to develop a better catalyst.
Thank you both for this clear explanation. I wish you also good luck with your future research and keep us updated on the progress!
Want to read the paper? You can find it here: Titanium-catalyzed esterification reactions: beyond Lewis acidity, ChemCatChem, 2020, 12, 5229-5235.