29 June 2022
Photocathodes could be more efficient
Photoelectrochemical cells are promising for the conversion of sunlight into fuel, for example water into hydrogen, or CO2 into organic molecules. To realize this, a higher efficiency of the photocathode, often based on NiO, is needed. An important and unknown question is the role of water molecules adsorbed on the NiO surface. Kaijian Zhu performs research on the effects of this adsorption.
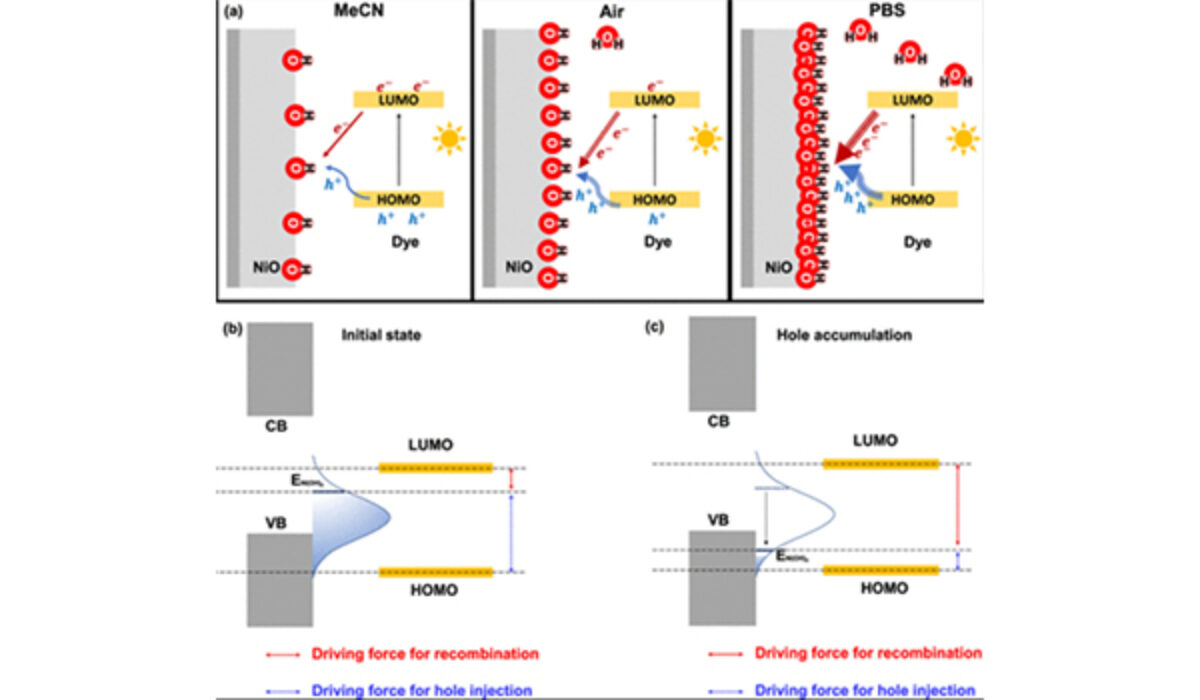
In this work we studied the light-induced processes occurring at the photocathode/electrolyte interface by advanced ultrafast spectroscopy. We show that hydroxyl groups formed at the NiO/water interface not only promote charge transfer between NiO and dye, but also increase the rate of charge recombination. Both processes are considerably slower when the photocathode is exposed to acetonitrile, while intermediate behavior is observed in air. This study shows that more efficient photocathodes can be developed by optimizing the amount of surface hydroxyl groups.
About the research
The research has been carried out by Kaijian Zhu, PhD student in the team of Dr. Annemarie Huijser, Associate Professor in the Photocatalytic Synthesis Group at the University of Twente. The project is part of the ARC CBBC. Within the ARC CBBC, the University of Twente and industrial partner Shell, among others, collaborate, in this project within the central theme ‘energy transition’.
The article “Dual Role of Surface Hydroxyl Groups in the Photodynamics and Performance of NiO-Based Photocathodes”, door Kaijian Zhu, Sean K. Frehan, Guido Mul and Annemarie Huijser is recently published in Journal of the American Chemical Society. Click here to read the article.