10 August 2022
ARC CBBC researcher Eva Blokker (VU Amsterdam) recently published her research in Angewandte Chemie. The team, supervised by VU Amsterdam Professor Matthias Bickelhaupt and in collaboration with chemical firm Nouryon, made a groundbreaking discovery that changes the way we look at chemistry. But what is this discovery?
In chemistry, radical stability is a major topic. Learning and predicting about radical stability is important in order to improve the industrial processes in which these chemical species are used.
State of the art
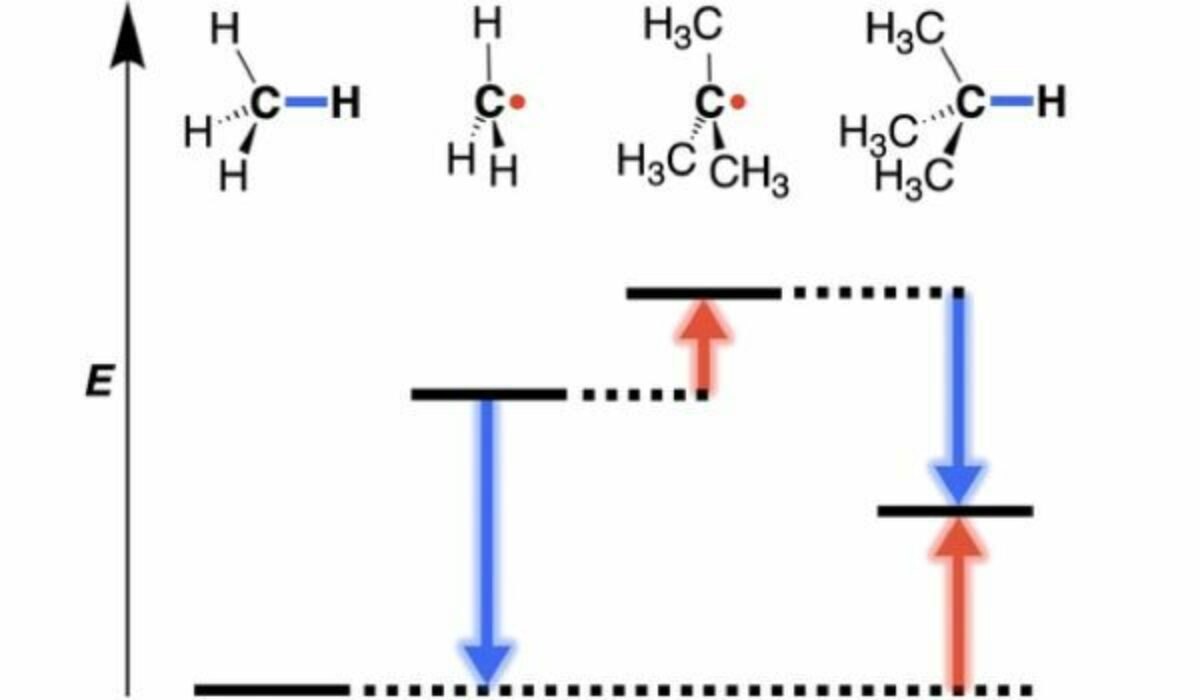
The state-of-the-art in physical organic chemistry is that alkyl radicals are stabilized upon an increase in their degree of methyl substitution from methyl < primary < secondary < tertiary. This increase in radical stability would be the underlying cause for the decrease in C–H bond strength along the series of methane (H3C–H), ethane (MeH2C–H), propane (Me2HC–H), and 2-methylpropane (Me3C–H). However, Blokker’s research proves this assumption wrong.
New findings
Blokker and colleagues investigated how methyl groups affect the stability of alkyl radicals and the corresponding alkanes. They reveal that radical stability decreases, and not increases, from methyl to primary to secondary to tertiary. This is not conflicting with the fact that the C–H bond becomes weaker along the series methane, ethane, propane, and 2-methylpropane. The resolution to this seeming contradiction is that the alkane is destabilized even more upon methyl substitution than the corresponding radical. This fundamental discovery on radical stability is groundbreaking, and will rewrite the textbooks for the future.
We would like to congratulate Blokker and her colleagues with this excellent contribution to the ever-changing landscape of science.
Click here to read the communication.