Electrochemical carbon-carbon coupling of CO2 has the potential to utilize this undesired gas. Unfortunately, CO2 reduction often suffers from sluggish kinetics and low selectivity due to many required electron transfer steps. However, oxalate synthesis only requires two electron transfers at the cost of a high overpotential. We try to reduce the overpotential to make oxalate synthesis an industrially viable CO2 reduction reaction.
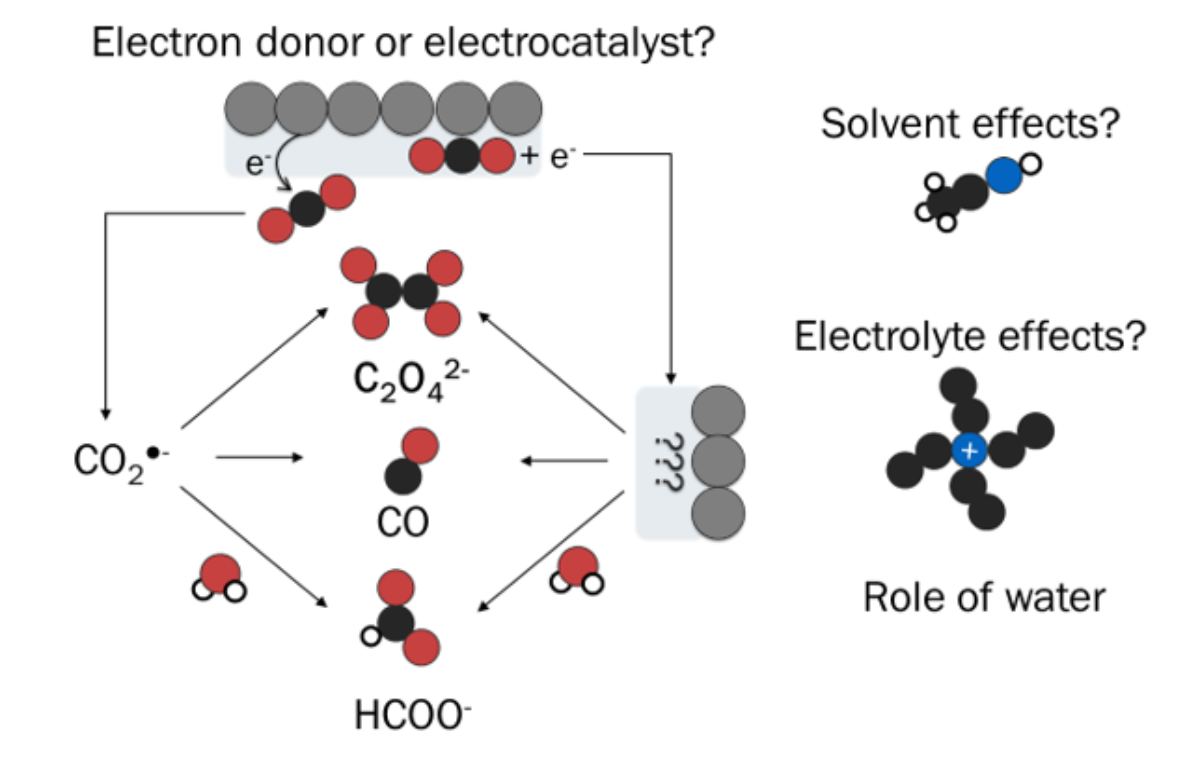
Electrochemical CO2 reduction is a promising technology for converting carbon dioxide into valuable chemicals and fuels. In aqueous solutions, CO2 reduction is well studied and continues as an active area of research. However, oxalate synthesis requires non-aqueous solvents because the presence of water promotes formate and hydrogen formation. In water free solvents, oxalate is formed by the coupling of two CO2 radical anions. The electrochemistry in non-aqueous solvents is not well established and defined posing the first challenge of the project. Furthermore, the generation of the radical anions requires large overpotentials. Traditionally, chemically inactive catalysts, as lead, are used to supress side reactions while generating these reactive species. The method requires large overpotentials which is energy inefficient. Therefore, the reaction might be improved by lowering the overpotential with an active electrocatalyst. This project sets out to find those catalysts from the bottom up. We aim to discover the catalytic pathways of oxalate synthesis and use this knowledge to improve the reaction efficiency.

