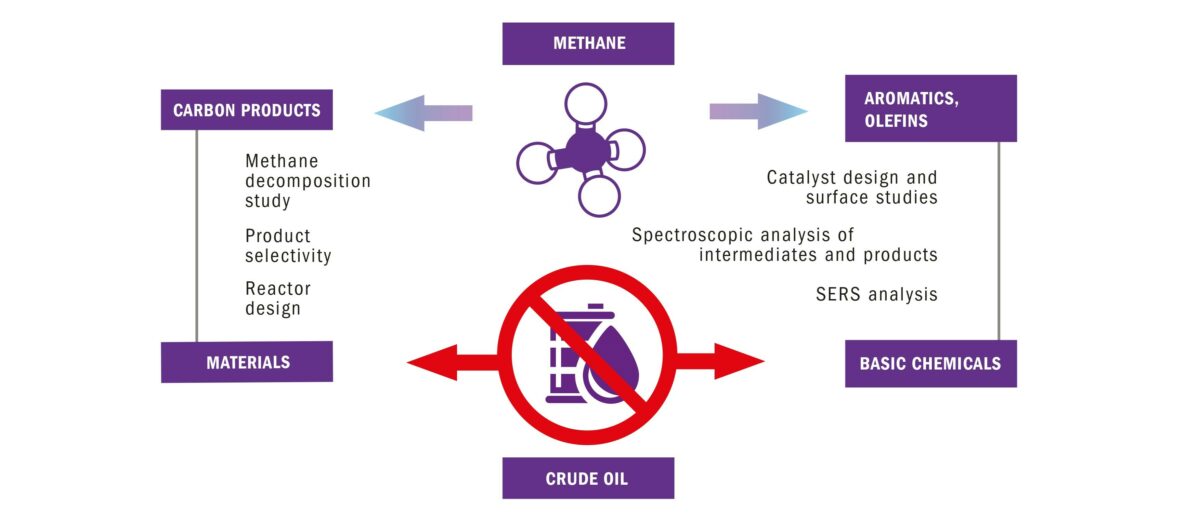
Methane has tremendous potential as a chemical feedstock. It is an abundant and cheap carbon source compared to other fossil resources, such as crude oil and coals. In this project, our research team is working on the conversion of methane into ethylene, hydrogen, aromatics and carbon materials, in both an energy- and atom-efficient way.
Small Molecule Activation: Pyrolytic upgrading of methane to ethylene, aromatics and carbon materials
Currently, commodity materials such as plastics are being made from crude oil. Crude oil consists of long hydrocarbon molecules, which are cracked into shorter hydrocarbon building blocks. Methane, however, is already a hydrocarbon that consists of just one carbon atom. Therefore, using methane as a base chemical would save much energy compared to the cracking process used to convert crude oil into building blocks. Also, methane being a greenhouse gas and a waste, its re-use as feedstock becomes essential to have a better control on our waste system.
Activating methane with a full control on the products made is a very challenging task requiring so far harsh conditions. Managing these process conditions is crucial for success and for enabling the use of such approach on a large scale. In this project, our focus points is therefore to develop improved catalysts and to increase our fundamental understanding of active sites and related reaction mechanisms, by using advanced characterization methods.
Methane instead of crude oil
Currently, carbon materials such as plastics are being made from crude oil. Crude oil consists of long hydrocarbon molecules, which are cracked into shorter hydrocarbon building blocks. Methane, however, is a hydrocarbon that consists of just one carbon atom. Using methane as a base chemical would save much energy that is currently needed in the cracking process.
Full control over the products
However, for methane, it is challenging to activate C-H bonds in such a way that there is full control over the products. Managing these harsh process conditions is crucial for success and therefore one of our focus points. Furthermore, we want to develop improved catalysts and to increase our fundamental understanding of active sites and related reaction mechanisms, by using advanced characterization methods.
Significant step in energy transition
Despite the clear potential of methane, efficient and industrially applicable direct methane-to-products conversions are not yet available for the chemical industry. The project will specifically explore the activation and chemistry of methane as a first and significant step in energy transition efforts, preceding work on other small and stable molecules, notably CO2 and N2.
Suitable solid catalysts
Within this multilateral project, we are exploring suitable solid catalysts for the pyrolytic conversion of methane into olefins, aromatics and structured carbons, all valuable chemical base materials. Not only do we wish to design new or improved catalyst materials, we also seek to increase our fundamental knowledge. For example knowledge on active catalytic sites and reaction mechanisms, on the underlying principles by which activity can be steered, on the product selectivity and catalyst stability and on effective catalyst-reactor design combinations.
Evaluate performance in real time
To this purpose, we will develop new characterization tools that allow us to evaluate the catalytic performance in real time. This will provide understanding of the activation mechanisms of methane and will allow us to follow the chemical conversion chemistry under severe reaction conditions, including its time- and composition dependence. The initial structure and composition of the newly developed catalyst will be related to (time-dependent) actual structure, surface composition and performance under reaction conditions.